癌癥治療新靶點(diǎn):FAP——從腫瘤微環(huán)境調(diào)控到診療一體化的突破之路
日期:2025-11-13 16:58:38
成纖維細(xì)胞活化蛋白(FAP)作為腫瘤微環(huán)境的核心調(diào)控因子,正成為癌癥診療領(lǐng)域的關(guān)鍵突破口。本文系統(tǒng)解析FAP在腫瘤進(jìn)展、免疫逃逸及纖維化疾病中的雙重作用機(jī)制,全面梳理靶向FAP的雙特異性抗體、CAR-T療法等前沿藥物研發(fā)進(jìn)展,探討其從基礎(chǔ)研究到臨床轉(zhuǎn)化的潛在價(jià)值,為腫瘤治療策略優(yōu)化提供新視角。
1. FAP研究的背景與意義
成纖維細(xì)胞活化蛋白(Fibroblast Activation Protein, FAP)是一種II型跨膜絲氨酸蛋白酶,在正常生理狀態(tài)下表達(dá)較低,但在多種病理?xiàng)l件下,尤其是在腫瘤微環(huán)境中,其表達(dá)顯著上調(diào),因此引起了廣泛關(guān)注 [1-4]。FAP最初因其在活化成纖維細(xì)胞上的表達(dá)而得名,并在多種疾病中,特別是纖維化、炎癥和癌癥中發(fā)揮重要作用 [4]。作為一種蛋白水解酶,F(xiàn)AP能夠切割細(xì)胞外基質(zhì)(ECM)中的蛋白質(zhì),影響細(xì)胞間的相互作用和組織重塑,進(jìn)而促進(jìn)疾病的發(fā)展 [1]。
FAP的表達(dá)模式使其成為潛在的生物標(biāo)志物和治療靶點(diǎn)。研究表明,F(xiàn)AP在超過90%的癌癥類型(如乳腺癌、胰腺癌、食管癌和肺癌)中的癌癥相關(guān)成纖維細(xì)胞(CAFs)上高度表達(dá),而在健康成人組織中幾乎不表達(dá) [2][3][5][6]。這種病理特異性表達(dá)賦予了FAP作為癌癥診斷和治療靶點(diǎn)的巨大潛力 [6]。
2. FAP的生物學(xué)特性、結(jié)構(gòu)與生理功能
FAP具有獨(dú)特的酶學(xué)活性,能夠特異性識別和降解細(xì)胞外基質(zhì)中的富含脯氨酸的成分(如膠原蛋白和明膠)[1][8]。在正常成人組織中,F(xiàn)AP的表達(dá)水平通常較低,僅在胚胎發(fā)育、創(chuàng)傷愈合等特定生理過程中呈現(xiàn)上調(diào) [1][4]。然而,在多種病理?xiàng)l件下,尤其是在腫瘤微環(huán)境中,F(xiàn)AP的表達(dá)顯著上調(diào),并成為癌癥相關(guān)成纖維細(xì)胞的標(biāo)志物 [2][5]。
2.1 FAP的結(jié)構(gòu)與酶學(xué)特性
FAP屬于二肽基肽酶(Dipeptidyl Peptidase, DPP)家族,是一種具有脯氨酸特異性的后脯氨酰蛋白水解酶 [10][8]。其酶學(xué)活性使其能夠切割多種細(xì)胞外基質(zhì)成分,參與組織重塑和病理進(jìn)程 [1]。在正常組織中,F(xiàn)AP的表達(dá)較低,但在胚胎發(fā)育、創(chuàng)傷修復(fù)等過程中會短暫上調(diào),以支持組織修復(fù) [4]。
2.2 FAP在正常組織中的表達(dá)與生理作用
盡管FAP在正常成人組織中表達(dá)水平較低,但在一些生理過程中,如胚胎發(fā)育和創(chuàng)傷愈合等,F(xiàn)AP會暫時性地上調(diào),并發(fā)揮重要的生理作用 [1][4]。FAP通過促進(jìn)成纖維細(xì)胞的遷移和增殖,以及參與細(xì)胞外基質(zhì)的重塑,支持組織的再生和修復(fù) [4]。這種受控的表達(dá)和功能展示了FAP在維持組織穩(wěn)態(tài)中的關(guān)鍵作用 [4]。
3. FAP在疾病中的作用機(jī)制與信號通路
FAP在多種疾病,尤其是腫瘤中的作用,主要通過細(xì)胞外基質(zhì)的重塑和細(xì)胞內(nèi)信號傳導(dǎo)的調(diào)控,影響腫瘤細(xì)胞的生物學(xué)行為。FAP在腫瘤微環(huán)境中的表達(dá)促進(jìn)了癌癥細(xì)胞的侵襲、轉(zhuǎn)移和治療抵抗。FAP不僅通過其酶學(xué)活性降解細(xì)胞外基質(zhì),還通過激活多個關(guān)鍵信號通路,調(diào)控細(xì)胞增殖、存活、遷移和免疫逃逸 [10][11]。
3.1 FAP與細(xì)胞外基質(zhì)重塑及細(xì)胞粘附
FAP在腫瘤微環(huán)境中通過降解細(xì)胞外基質(zhì)中的成分,影響腫瘤細(xì)胞與基質(zhì)的粘附和遷移。研究表明,F(xiàn)AP通過整合素家族成員與細(xì)胞外基質(zhì)的相互作用,激活黏著斑激酶(FAK)信號通路,促進(jìn)腫瘤的侵襲和轉(zhuǎn)移 [10]。在非小細(xì)胞肺癌(NSCLC)中,F(xiàn)AP通過調(diào)控整合素與ECM成分的結(jié)合,進(jìn)一步促進(jìn)腫瘤轉(zhuǎn)移 [11]。此外,F(xiàn)AP與整合素之間的相互作用改變了腫瘤細(xì)胞的粘附強(qiáng)度和遷移能力,從而為腫瘤細(xì)胞的侵襲提供了有利條件 [11]。
3.2 FAP介導(dǎo)的細(xì)胞內(nèi)信號傳導(dǎo)與細(xì)胞行為調(diào)控
FAP通過多種信號通路調(diào)控腫瘤細(xì)胞的增殖、存活和遷移。例如,在結(jié)直腸癌(CRC)中,F(xiàn)AP通過激活腫瘤壞死因子受體2(TNFR2)/Akt或ERK信號通路,促進(jìn)成纖維細(xì)胞的增殖和遷移 [17]。FAP還通過與Yes關(guān)聯(lián)蛋白(YAP1)的相互作用,調(diào)控細(xì)胞增殖和免疫逃逸。例如,在高級別漿液性卵巢癌(HGSOC)中,F(xiàn)AP+ CAFs通過YAP1依賴的機(jī)制,抑制CD8+ T細(xì)胞的細(xì)胞毒性,降低免疫反應(yīng),從而導(dǎo)致患者預(yù)后不良 [15]。
FAP還與轉(zhuǎn)化生長因子-β(TGF-β)通路密切相關(guān),TGF-β通過FAP/VCAN軸促進(jìn)膀胱癌細(xì)胞的上皮-間充質(zhì)轉(zhuǎn)化(EMT),增強(qiáng)腫瘤細(xì)胞的侵襲性 [16]。這些發(fā)現(xiàn)揭示了FAP在腫瘤微環(huán)境中的重要作用,尤其是在腫瘤細(xì)胞增殖和免疫逃逸中的多重機(jī)制 [11][17]。
3.3 FAP與腫瘤微環(huán)境的相互作用
FAP陽性成纖維細(xì)胞(CAFs)在腫瘤微環(huán)境中發(fā)揮多重作用,尤其在免疫逃逸、治療抵抗和腫瘤轉(zhuǎn)移中起著至關(guān)重要的作用。FAP+ CAFs通過分泌免疫抑制因子,如IL-8,誘導(dǎo)腫瘤細(xì)胞的放療抵抗 [22]。此外,F(xiàn)AP+ CAFs還通過改變細(xì)胞外基質(zhì)的成分,阻止免疫細(xì)胞的浸潤,形成“冷”腫瘤微環(huán)境,進(jìn)一步促進(jìn)腫瘤的免疫逃逸 [20]。
FAP通過與CXCL12等因子的相互作用,促進(jìn)CAF的免疫抑制功能。例如,在胃癌中,F(xiàn)AP+ CAFs通過分泌骨膜素(POSTN)促進(jìn)巨噬細(xì)胞的趨化,并通過Akt信號通路激活免疫檢查點(diǎn)抑制劑(ICB)的抵抗 [21]。這些研究表明,F(xiàn)AP在腫瘤微環(huán)境中的作用不僅局限于ECM重塑,還通過免疫調(diào)節(jié)、細(xì)胞遷移等機(jī)制推動腫瘤的惡性轉(zhuǎn)化 [21]。
3.4 FAP在非腫瘤疾病中的作用
FAP在非腫瘤性疾病中的作用逐漸受到關(guān)注,特別是在纖維化和慢性炎癥性疾病中。例如,在心肌缺血再灌注損傷(I/R)后,F(xiàn)AP在心肌成纖維細(xì)胞中的表達(dá)顯著上調(diào),促進(jìn)了心肌纖維化 [19]。類似地,在肝纖維化中,F(xiàn)AP的表達(dá)水平與肝星狀細(xì)胞的激活密切相關(guān),并且FAP-α作為纖維化的標(biāo)志物在臨床中具有重要的診斷價(jià)值 [18]。
4. FAP靶向藥物研發(fā)進(jìn)展
在腫瘤領(lǐng)域,針對FAP的放射性藥物研發(fā)非常活躍,已有多條管線處于臨床階段,并且正從單純的診斷或治療,向“診療一體化”的方向發(fā)展。另外,小分子化藥、多肽偶聯(lián)核素、雙特異性抗體、細(xì)胞治療藥物等多種藥物類型在研,部分雙抗及CAR-T在研管線列舉如下表:
| 藥物 | 作用機(jī)制 | 藥物類型 | 在研適應(yīng)癥(疾病名) | 在研機(jī)構(gòu) | 最高研發(fā)階段 |
|---|---|---|---|---|---|
| RG7826 | 4-1BB激動劑 | FAP拮抗劑 | 雙特異性抗體 | 結(jié)直腸癌 | Roche Holding AG | 臨床1/2期 |
| RO-7567132 | FAP拮抗劑 | TNFRSF3 agonists | 雙特異性抗體 | 晚期惡性實(shí)體瘤 | 局部晚期惡性實(shí)體瘤 | 轉(zhuǎn)移性實(shí)體瘤 | Hoffmann-La Roche Ltd. | 臨床1期 |
| RO-7300490 | CD40L刺激劑 | FAP調(diào)節(jié)劑 | 雙特異性抗體 | 局部晚期惡性實(shí)體瘤 | Hoffmann-La Roche, Inc. | 臨床1期 |
| BMS-986484 | CD40抑制劑 | FAP拮抗劑 | 雙特異性抗體 | 晚期惡性實(shí)體瘤 | Bristol Myers Squibb Co. | 臨床1期 |
| SHR-7367 | CD40激動劑 | FAP拮抗劑 | 雙特異性抗體 | 晚期癌癥 | 晚期惡性實(shí)體瘤 | KRAS G12D突變實(shí)體瘤 | 上海恒瑞醫(yī)藥有限公司 | 臨床1期 |
| BI-765179 | 4-1BB激動劑 | FAP調(diào)節(jié)劑 | 雙特異性抗體 | 晚期惡性實(shí)體瘤 | 局部晚期惡性實(shí)體瘤等 | Boehringer Ingelheim International GmbH | Boehringer Ingelheim GmbH | 臨床1期 |
| GEN1057 | DR4激動劑 | FAP拮抗劑 | 雙特異性抗體 | 晚期惡性實(shí)體瘤 | 轉(zhuǎn)移性實(shí)體瘤 | 實(shí)體瘤 | Genmab BV | Genmab, Inc. | 臨床1期 |
| AGEN1721 | FAP調(diào)節(jié)劑 | TGF-β調(diào)節(jié)劑 | 雙特異性抗體 | 乳腺癌 | 結(jié)直腸癌 | Agenus, Inc. | 臨床前 |
| 四價(jià)FAPxCD40雙抗(恒瑞醫(yī)藥) | CD40激動劑 | FAP調(diào)節(jié)劑 | 雙特異性抗體 | 多價(jià)疫苗 | 腫瘤 | 江蘇恒瑞醫(yī)藥股份有限公司 | 臨床前 |
| FAP靶向的CAR-T(Capstan Therapeutics) | FAP拮抗劑 | CAR-T | 纖維化 | Capstan Therapeutics, Inc. | 臨床前 |
| FAP CAR T cell therapy(Acuitas) | FAP拮抗劑 | CAR-T | 心臟衰竭 | Acuitas Therapeutics, Inc. | 臨床前 |
| FAP靶向CAR-T(Genethon) | FAP拮抗劑 | CAR-T | 纖維化 | 杜氏肌營養(yǎng)不良癥 | Genethon | 臨床前 |
| 抗FAP/TGF-PRII抗體(Merus) | FAP拮抗劑 | TGFBR2抑制劑 | 雙特異性抗體 | 腫瘤 | Incyte Corp. | Merus NV | 臨床前 |
| M-300 (Mestag) | FAP調(diào)節(jié)劑 | TNFRSF3 agonists | 雙特異性抗體 | 實(shí)體瘤 | Mestag Therapeutics Ltd. | 臨床前 |
| 抗OX40/FAP-α雙抗(羅氏) | FAP調(diào)節(jié)劑 | OX40激動劑 | 雙特異性抗體 | 腫瘤 | F. Hoffmann-La Roche Ltd. | 臨床前 |
| OPTF01 | FAP拮抗劑 | CAR-T | 膠質(zhì)母細(xì)胞瘤 | Optieum Biotechnologies, Inc. | 臨床前 |
5. FAP研究工具
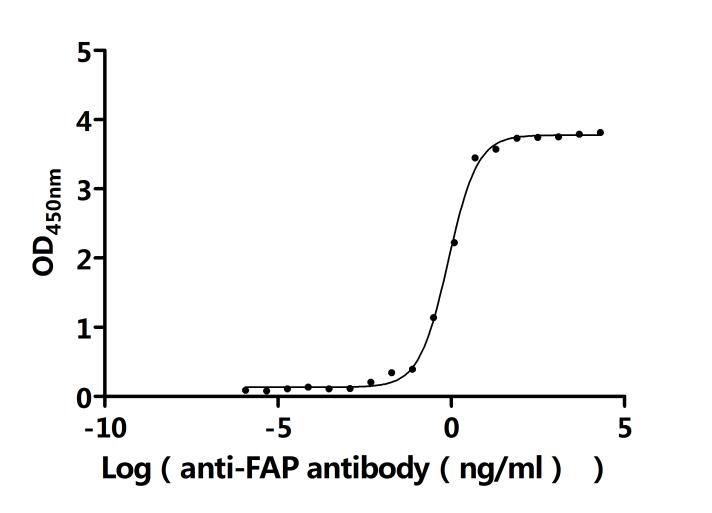
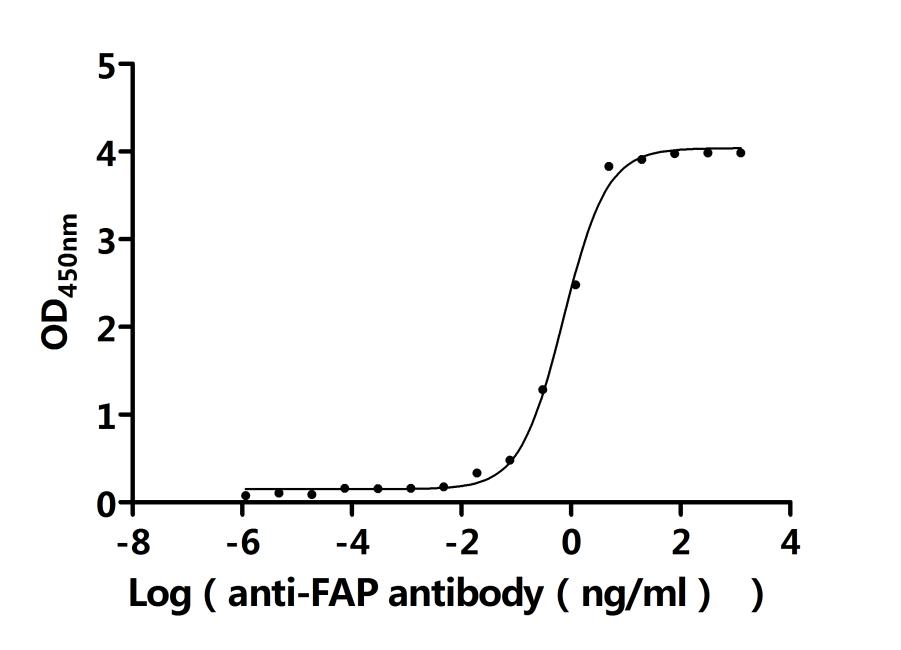
FAP作為腫瘤微環(huán)境中的關(guān)鍵靶點(diǎn),其在腫瘤的侵襲、轉(zhuǎn)移、免疫逃逸以及耐藥性中發(fā)揮重要作用。華美生物提供FAP重組蛋白、抗體及ELISA試劑盒產(chǎn)品,助力您開發(fā)特異性靶向FAP的藥物,探索其在腫瘤治療中的應(yīng)用潛力。
參考文獻(xiàn):
[1] Rasmus S Pedersen, M. Karsdal, N. Willumsen.(2024). Abstract 4287: Serological quantification of fibroblast activation protein (FAP) cleaved type III collagen: A biomarker for FAP activity.
[2] Sebastian Dziadek, A. Kraxner, Wei-Yi Cheng, Tai-Hsien Ou Yang, Mike Flores, Noah Theiss, T. Tsao, Emilia Andersson, S. V. Harring, Ann-Marie E. Br?ske, Maurizio Ceppi, Volker Teichgr?ber, Jehad Charo.(2024). Comprehensive analysis of fibroblast activation protein expression across 23 tumor indications: insights for biomarker development in cancer immunotherapies.
[3] K. Hartmann, Merel van Gogh, P. C. Freitag, Florian Kast, Gabriela Nagy-Davidescu, L. Borsig, A. Plückthun.(2022). FAP-retargeted Ad5 enables in vivo gene delivery to stromal cells in the tumor microenvironment.
[4] Zihan Wang, Jinping Wang, Tianyi Lan, Liubo Zhang, Zeran Yan, N. Zhang, Yuan Xu, Qing-wen Tao.(2023). Role and mechanism of fibroblast-activated protein-α expression on the surface of fibroblast-like synoviocytes in rheumatoid arthritis.
[5] Spencer D. Lindeman, Jack Higgins.(2024). Abstract 6026: A novel lutetium-177 radioligand therapy targeting FAP has potent antitumor activity in xenograft cancer model.
[6] Ruchi Shah, Katherine A. Johnson, Anna E L Lippert, Sean G Kraus, Philip B. Emmerich, Cheri A Pasch, Wei Zhang, K. Matkowskyj, Aaron M LeBeau, D. Deming.(2024). Cancer-Associated Fibroblast Proteins as Potential Targets against Colorectal Cancers.
[7] Mengxin Xu, Pu Zhang, J. Ding, Junyi Chen, L. Huo, Zhibo Liu.(2021). Albumin Binder–Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy.
[8] Layne N. Raborn, Z. Michel, Michael T. Collins, Alison M Boyce, L. F. de Castro.(2024). Fibroblast Activation Protein Is Expressed by Altered Osteoprogenitors and Associated to Disease Burden in Fibrous Dysplasia.
[9] L. Loureiro, Lydia Hoffmann, Christin Neuber, Luise Rupp, C. Arndt, A. Kegler, M. Kubeil, Christoph E Hagemeyer, Holger Stephan, Marc Schmitz, A. Feldmann, M. Bachmann.(2023). Immunotheranostic target modules for imaging and navigation of UniCAR T-cells to strike FAP-expressing cells and the tumor microenvironment.
[10] Yentl Van Rymenant, Anke de Groot, Laura Dirkx, Emile Verhulst, Joni De Loose, Isabel Pintelon, Tias Verhezen, J. De Waele, Sofie Thys, O. De Wever, Muhammet Tanc, G. Caljon, Pieter Van der Veken, Ingrid De Meester.(2025). FAP on human NK cells: insights from NK cell activation and crosstalk with cancer-associated fibroblasts.
[11] Lirong Gao, Anqi Wang, Yuling Chen, Xin Cai, Yue Li, Jian Zhao, Yang Zhang, Weijie Zhang, Jianjie Zhu, Yuanyuan Zeng, Zeyi Liu, Jianyang Huang.(2023). FTO facilitates cancer metastasis by modifying the m6A level of FAP to induce integrin/FAK signaling in non-small cell lung cancer.
[12] Patrizio Ansalone.(2014). Electrostatic Affinities and Binding Kinetics among α2I Integrin Domains, Divalent Cations and 21-mer Collagen Fragment.
[13] Samuel Bell, Eugene M. Terentjev.(2016). Focal adhesion kinase - the reversible molecular mechanosensor.
[14] Min Li, Xue Cheng, Rong Rong, Yan-ping Gao, Xiuwu Tang, Youguo Chen.(2020). High expression of fibroblast activation protein (FAP) predicts poor outcome in high-grade serous ovarian cancer.
[15] Monika Licaj, R. Mhaidly, Y. Kieffer, H. Croizer, C. Bonneau, A. Meng, L. Djerroudi, Kevin Mujangi-Ebeka, H. Hocine, B. Bourachot, Ilaria Magagna, Renaud Leclère, Léa Guyonnet, Mylène Bohec, Coralie Guérin, S. Baulande, M. Kamal, C. le Tourneau, Fabrice Lécuru, Véronique Becette, Roman Rouzier, A. Vincent-Salomon, Géraldine Gentric, F. Mechta-Grigoriou.(2024). Residual ANTXR1+ myofibroblasts after chemotherapy inhibit anti-tumor immunity via YAP1 signaling pathway.
[16] Q. Ping, Chunhui Wang, Xin Cheng, Yiming Zhong, R. Yan, Meng Yang, Yunqiang Shi, Xiangmeng Li, Xiao Li, Wenwen Huang, Liqiong Wang, Xiaofang Bi, Libing Hu, Yang Yang, Yingbao Wang, R. Gong, Jun Tan, Rui Li, Hui Li, Jian Li, Wenju Wang, Ruhong Li.(2023). TGF-β1 dominates stromal fibroblast-mediated EMT via the FAP/VCAN axis in bladder cancer cells.
[17] Linlin Wang, Dong Yang, Jing Tian, Aiqin Gao, Yihang Shen, X. Ren, Xia Li, G. Jiang, Taotao Dong.(2017). Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer.
[18] Ruifang Li, Yifan Tai, Xinyan Zhang, Zhen Liu, Haipeng Si, Deling Kong, Lili Zhao, Jia Li, Adam C. Midgley.(2025). Tissue‐Microenvironment‐Responsive Self‐Assembling Peptide Nanoshells Boost Pirfenidone Efficacy in the Treatment of Liver Fibrosis.
[19] Jiawan Wang, Heng Du, Wanrun Xie, Jinmiao Bi, Hao Zhang, Xu Liu, Yuhan Wang, Shaolong Zhang, Anhua Lei, Chuting He, Hailong Yuan, Jiahe Zhang, Yujing Li, Pengfei Xu, Siqi Liu, Yanan Zhou, Jianghua Shen, Jingdong Wu, Yihong Cai, Chaofan Yang, Zeya Li, Y. Liang, Yang Zhao, Jin Zhang, Moshi Song.(2024). CAR-Macrophage Therapy Alleviates Myocardial Ischemia-Reperfusion Injury.
[20] Shipra Das, J. Valton, P. Duchateau, L. Poirot.(2023). Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy.
[21] Tingting You, Huijing Tang, Wenjing Wu, Jin Gao, Xuechun Li, Ningning Li, Xiuxiu Xu, Jiazhang Xing, Hui Ge, Yi Xiao, Junchao Guo, Bin Wu, Xiaoyi Li, Liangrui Zhou, Lin Zhao, C. Bai, Qin Han, Zhao Sun, R. Zhao.(2023). POSTN Secretion by Extracellular Matrix Cancer-Associated Fibroblasts (eCAFs) Correlates with Poor ICB Response via Macrophage Chemotaxis Activation of Akt Signaling Pathway in Gastric Cancer.
[22] Weiqiang Huang, Longshan Zhang, Mi Yang, Xixi Wu, Xiaoqing Wang, Wenqi Huang, Lu Yuan, Hua Pan, Yin Wang, Zici Wang, Yuting Wu, Jihong Huang, Huazhen Liang, Shaoqun Li, Liwei Liao, Laiyu Liu, J. Guan.(2020). Cancer-associated fibroblasts promote the survival of irradiated nasopharyngeal carcinoma cells via the NF-κB pathway.